Hai............. how is going..........
Sorry for not posing any article since last three days.......
But.....
Today I came with very interesting article and
Here I am trying to clarify so many doubts about " Forced degradation study", and explain the practical approach in different cases.
a) To know the degradation pathways of drug substances and drug products.
b) To differentiate degradation products that are related to drug products from those that are generated from placebo in a formulation.
c) To findout the structure of degradation products.
d) To determine the intrinsic stability of a drug substance in formulation.
e) To reveal the degradation mechanisms such as hydrolysis, oxidation, thermolysis or photolysis of the drug substance and drug product.
f) To establish stability indicating nature of a developed method.
g) To understand the chemical properties of drug molecules.
h) To generate more stable formulations.
i) To produce a degradation profile similar to that of what would be observed in a formal stability study under ICH conditions.
j) To solve stability-related problems.
3) When Forced Degradation Studies Do.?
a) During Formulation studies, forced degradation studies are useful to establish stability indicating nature of developed analytical method, to compare pre manufacturing and post manufacturing changes.
b) During Pre-clinical studies, forced degradation studies are useful to identify degradants, toxic components like-body conjugates, etc...
Stability and forced degradation requirements in ICH quality guidelines
ICH guideline title Comments
Q1B: Photostability testing of new drug substances and products Provides guidance for the generation of photostability studies to support submission in registration applications for new molecular entities.
Q2(R1): Validation of analytical procedures: text and methodology Forced degradation studies are required for analytical method validation to demonstrate test specificity ( stability indicating nature)
Q3A(R2): Impurities in new drug substances
Q3B(R2): Impurities in new drug products Recommends the use of appropriate stress conditions for validation of analytical procedures applicable to new chemical entities.
Biological/biotechnological products not covered in the guidelines.
Q5C: Stability testing of biotechnological/biological products Recommends the use of accelerated and stress conditions to support the
establishment of expiration date and to support product comparability.
Selection of stress conditions to be conducted on a case-by-case basis.
Q5E: Comparability of biotechnological/biological products subject
to changes in their manufacturing process
The manufacturing process and its changes may potentially produce different degradants and degradation pathways. Recommends forced degradation studies to establish potential product differences in the degradation pathways.
Q6B: Test procedures and acceptance criteria for biotechnological/
biological products Forced degradation studies can help to understand process related
degradants/impurities; justify and rationalize to meet the requirements of setting up the acceptance criteria based on potency and the level of
product-related impurities.
Q8(R2): Pharmaceutical development Forced degradation studies usually include extended variations (ie,
temperature, pH, light, shear), container closure system compatibility, and suitability studies under normal and stressed storage conditions.
Q11: Development and manufacture of drug substance Forced degradation studies will provide product knowledge and fulfill
quality requirements.
The practical approach to the forced degradation depends on requirement of forced degradation study.
The most common type of Dedradations are....
1) Hydrolytic Degradation : Degradation with acids, bases and water etc...
2) Oxidative degradation : Degradation with oxidising agents like peroxide, potassium permanganate etc.....
3) Thermal Degradation : Degradation with heat
4) Photolytic Degradation : Degradation by exposing to UV, Visible light.
These are main degradation types and apart from that, metallic degradation also used in some cases.
Hydrolytic Degradation:
Hydrolysis is a solvolytic process in which drug reacts with water to yield breakdown products of different chemical compositions.
Acid Degradation: Hydrolysis under acidic condition.
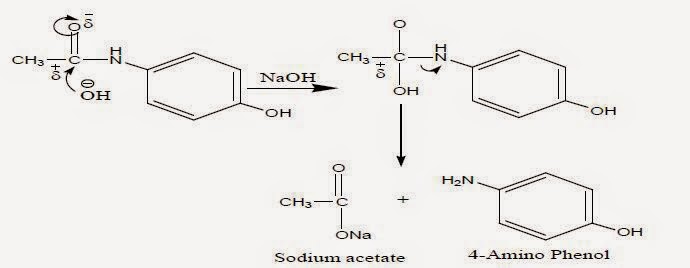
Above diagram shows how paracetamol undergoes hydrolysis in basic condition. Most common base used for base hydrolysis is Sodium hydroxide.
Most recommended range will be 0.1N to a maximum of 5N of base in case of sodium hydroxide. If concentration of base is more, it will impact badly on HPLC column.
Drug substances(DS), Drug product(DP) and Placebo were degraded with base to attain required amount of degradation. Neutralise the sample with same concentration of acid. Then follow procedure as per analytical methodology. Differentiate the peaks based on peaks obtained from DS, DP and placebo.
Note: If sample mass is more, first dissolve the sample mass in diluent then proceed for degradation. Our final goal is to degrade the analyte. If no degradation achieved even with the addition highly concentrated acid/base, then reflux the sample after adding acid/base, cool to room temperature and proceed as per analytical methodology.
Humidity Degradation: Hydrolysis under high humidity condition.
Humidity under different temperatures is one of the possible cause for degradation during stability studies. Excess humidity ( over 90%) should be applied to the samples to degrade.
Saturated solution of Sodium nitrate or potassium nitrate under vacuum can be used to produce excess humidity.
Water Degradation: Hydrolysis with water under heat condition.
Samples can undergo hydrolysis with water in presence of high temperatures.
Drug substances(DS), Drug product(DP) and Placebo were degraded with water at temperatures ( 70°C to 80°C) to attain required amount of degradation. Neutralise the sample with same concentration of acid. Then follow procedure as per analytical methodology. Differentiate the peaks based on peaks obtained from DS, DP and placebo.
Oxidative Degradation:
Oxidative Degradation: Oxidation under strong oxidizing agents.
Maximum recommended concentration is 10% hydrogen peroxide.
Drug substances(DS), Drug product(DP) and Placebo were degraded with oxidizing agent to attain required amount of degradation. Then follow procedure as per analytical methodology. Differentiate the peaks based on peaks obtained from DS, DP and placebo.
Problem with hydrogen peroxide: Some times a peak due to hydrogen peroxide will interfere with degradant peaks. In such cases, an alternative oxidizing agent can be used. e.g potassium permanganate.
Note: If sample mass is more, first dissolve the sample mass in diluent then proceed for degradation. Our final goal is to degrade the analyte.
Thermal Degradation:
Temperature is one of the major cause for degradation of pharmaceutical products in stability studies.
DS, DP and Placebo can be exposed to excess heat ( probably more heat than Accelerated condition-40°C/75%RH) to get required degradation.
Note: Samples should not be exposed more than to its boiling points.
Photolytic Degradation:
As per ICH Q1B guideline, Samples should be exposed to overall illumination of not less than 1.2 million lux hours to visible light and integrated UV energy of not less than 200 watts/square meter.
Calculate the intensity of light with lux meter and load samples to expose to visible and UV light. After completion of required time, prepare samples as per analytical methodlogy and anlyse.
" If samples were not degraded in any one or more of the above degradation studies, declare the sample is stable with respect to particular type of degradation. "
5) How much to be degraded .?
Now its a big question for all scientists that how much the sample to be degraded..?
This purely based on the purpose of degradation.
If forced degradation study is to elucidate the structure of degradation product, % degradation to be achieved is based on how the structure is determined.(structure can be determined by LC-MS, LC-MS/MS and NMR spectrometry etc....).
If forced degradation study is to prove an analytical method is stability indicating, then the % degradation should be not less than specification of Total impurities. How ever it is not fixed.
Excessive degradation may lead to secondary degradation (i.e degraded products may further degrade).
Hence a moderate degradation from 5% to 20% may be good choice.
Some scientists may say slightly more than 10% degradation is acceptable for stability indicating methods, because the specification to the % assay for major of the products is NLT 90% until unless justified.
"So that's it for today. I think after reading this, so many doubts may arise to the readers. so please post your questions as comments below.
My next topic will be " Stability indicating Methods "
Thanks and bye.........
V.Suresh.
Sorry for not posing any article since last three days.......
But.....
Today I came with very interesting article and
That is........
"Forced Degradation Study"
It is typical task to many scientists to perform Forced degradation study to the formulation products.
Here I am trying to clarify so many doubts about " Forced degradation study", and explain the practical approach in different cases.
Before going to practical approach I want to tell.......
What.............?
Why.............?
When...........?
How............?
How much........?
How much........?
1) What is Forced degradation.?
Forced degradation is a ..............
" process of increasing the Degradation rate of a material or product by the application of additional forces like Oxidation, Reduction, excess heat, excess humidity, more intense light etc......."
2) Why Forced degradation studies are required.?
b) To differentiate degradation products that are related to drug products from those that are generated from placebo in a formulation.
c) To findout the structure of degradation products.
d) To determine the intrinsic stability of a drug substance in formulation.
e) To reveal the degradation mechanisms such as hydrolysis, oxidation, thermolysis or photolysis of the drug substance and drug product.
f) To establish stability indicating nature of a developed method.
g) To understand the chemical properties of drug molecules.
h) To generate more stable formulations.
i) To produce a degradation profile similar to that of what would be observed in a formal stability study under ICH conditions.
j) To solve stability-related problems.
3) When Forced Degradation Studies Do.?
a) During Formulation studies, forced degradation studies are useful to establish stability indicating nature of developed analytical method, to compare pre manufacturing and post manufacturing changes.
b) During Pre-clinical studies, forced degradation studies are useful to identify degradants, toxic components like-body conjugates, etc...
c) During Clinical development, Forced degradation studies are useful to compare Pre-clinical and clinical quality.
Stability and forced degradation requirements in ICH quality guidelines
ICH guideline title Comments
Q1B: Photostability testing of new drug substances and products Provides guidance for the generation of photostability studies to support submission in registration applications for new molecular entities.
Q2(R1): Validation of analytical procedures: text and methodology Forced degradation studies are required for analytical method validation to demonstrate test specificity ( stability indicating nature)
Q3A(R2): Impurities in new drug substances
Q3B(R2): Impurities in new drug products Recommends the use of appropriate stress conditions for validation of analytical procedures applicable to new chemical entities.
Biological/biotechnological products not covered in the guidelines.
Q5C: Stability testing of biotechnological/biological products Recommends the use of accelerated and stress conditions to support the
establishment of expiration date and to support product comparability.
Selection of stress conditions to be conducted on a case-by-case basis.
Q5E: Comparability of biotechnological/biological products subject
to changes in their manufacturing process
The manufacturing process and its changes may potentially produce different degradants and degradation pathways. Recommends forced degradation studies to establish potential product differences in the degradation pathways.
Q6B: Test procedures and acceptance criteria for biotechnological/
biological products Forced degradation studies can help to understand process related
degradants/impurities; justify and rationalize to meet the requirements of setting up the acceptance criteria based on potency and the level of
product-related impurities.
Q8(R2): Pharmaceutical development Forced degradation studies usually include extended variations (ie,
temperature, pH, light, shear), container closure system compatibility, and suitability studies under normal and stressed storage conditions.
Q11: Development and manufacture of drug substance Forced degradation studies will provide product knowledge and fulfill
quality requirements.
4) How Forced degradation do.?(Practical approach)
The most common type of Dedradations are....
1) Hydrolytic Degradation : Degradation with acids, bases and water etc...
2) Oxidative degradation : Degradation with oxidising agents like peroxide, potassium permanganate etc.....
3) Thermal Degradation : Degradation with heat
4) Photolytic Degradation : Degradation by exposing to UV, Visible light.
These are main degradation types and apart from that, metallic degradation also used in some cases.
Hydrolytic Degradation:
Hydrolysis is a solvolytic process in which drug reacts with water to yield breakdown products of different chemical compositions.
Acid Degradation: Hydrolysis under acidic condition.
Above diagram shows how paracetamol undergoes hydrolysis in acidic condition. Most common acid used for acid hydrolysis is hydrochloric acid.
There are no specific guidance for how much concentration of acid to be taken for degradation. Hence most recommended range will be 0.1N to a maximum of 5N of acid in case of hydrochloric acid. If concentration of acid is more, it will impact badly on HPLC column.
Drug substances(DS), Drug product(DP) and Placebo were degraded with Acid to attain required amount of degradation. Neutralise the sample with same concentration of base. then follow procedure as per analytical methodology. Differentiate the peaks based on peaks obtained from DS, DP and placebo.
Base Degradation: Hydrolysis under Basic condition.

Above diagram shows how paracetamol undergoes hydrolysis in basic condition. Most common base used for base hydrolysis is Sodium hydroxide.
Most recommended range will be 0.1N to a maximum of 5N of base in case of sodium hydroxide. If concentration of base is more, it will impact badly on HPLC column.
Drug substances(DS), Drug product(DP) and Placebo were degraded with base to attain required amount of degradation. Neutralise the sample with same concentration of acid. Then follow procedure as per analytical methodology. Differentiate the peaks based on peaks obtained from DS, DP and placebo.
Note: If sample mass is more, first dissolve the sample mass in diluent then proceed for degradation. Our final goal is to degrade the analyte. If no degradation achieved even with the addition highly concentrated acid/base, then reflux the sample after adding acid/base, cool to room temperature and proceed as per analytical methodology.
Humidity Degradation: Hydrolysis under high humidity condition.
Humidity under different temperatures is one of the possible cause for degradation during stability studies. Excess humidity ( over 90%) should be applied to the samples to degrade.
Saturated solution of Sodium nitrate or potassium nitrate under vacuum can be used to produce excess humidity.
Water Degradation: Hydrolysis with water under heat condition.
Samples can undergo hydrolysis with water in presence of high temperatures.
Drug substances(DS), Drug product(DP) and Placebo were degraded with water at temperatures ( 70°C to 80°C) to attain required amount of degradation. Neutralise the sample with same concentration of acid. Then follow procedure as per analytical methodology. Differentiate the peaks based on peaks obtained from DS, DP and placebo.
Oxidative Degradation:
Oxidative Degradation: Oxidation under strong oxidizing agents.
Above diagram shows how paracetamol undergoes oxidation under strong oxidizing agents. Most common oxidising agent is Hydrogen peroxide.Maximum recommended concentration is 10% hydrogen peroxide.
Drug substances(DS), Drug product(DP) and Placebo were degraded with oxidizing agent to attain required amount of degradation. Then follow procedure as per analytical methodology. Differentiate the peaks based on peaks obtained from DS, DP and placebo.
Problem with hydrogen peroxide: Some times a peak due to hydrogen peroxide will interfere with degradant peaks. In such cases, an alternative oxidizing agent can be used. e.g potassium permanganate.
Note: If sample mass is more, first dissolve the sample mass in diluent then proceed for degradation. Our final goal is to degrade the analyte.
Thermal Degradation:
Temperature is one of the major cause for degradation of pharmaceutical products in stability studies.
DS, DP and Placebo can be exposed to excess heat ( probably more heat than Accelerated condition-40°C/75%RH) to get required degradation.
Note: Samples should not be exposed more than to its boiling points.
Photolytic Degradation:
As per ICH Q1B guideline, Samples should be exposed to overall illumination of not less than 1.2 million lux hours to visible light and integrated UV energy of not less than 200 watts/square meter.
Calculate the intensity of light with lux meter and load samples to expose to visible and UV light. After completion of required time, prepare samples as per analytical methodlogy and anlyse.
" If samples were not degraded in any one or more of the above degradation studies, declare the sample is stable with respect to particular type of degradation. "
5) How much to be degraded .?
Now its a big question for all scientists that how much the sample to be degraded..?
This purely based on the purpose of degradation.
If forced degradation study is to elucidate the structure of degradation product, % degradation to be achieved is based on how the structure is determined.(structure can be determined by LC-MS, LC-MS/MS and NMR spectrometry etc....).
If forced degradation study is to prove an analytical method is stability indicating, then the % degradation should be not less than specification of Total impurities. How ever it is not fixed.
Excessive degradation may lead to secondary degradation (i.e degraded products may further degrade).
Hence a moderate degradation from 5% to 20% may be good choice.
Some scientists may say slightly more than 10% degradation is acceptable for stability indicating methods, because the specification to the % assay for major of the products is NLT 90% until unless justified.
"So that's it for today. I think after reading this, so many doubts may arise to the readers. so please post your questions as comments below.
My next topic will be " Stability indicating Methods "
Thanks and bye.........
V.Suresh.


hello Sir,its very good initative....very nice...
ReplyDeleteThanks for compliment.... Please visit my blog regularly for useful information....
ReplyDelete